OUR OFFERINGS
Expect great science.
It’s in our DNA.
ExcellGene’s reliable process and exceptional CHO cell hosts are the result of over twenty years of innovation and fine-tuning.
OUR SERVICES
A uniquely
science-driven CDMO
Since 2001, ExcellGene has developed proprietary technologies and know-how that are key for the delivery of its clients’ projects on a timely manner and with a high degree of customization.
CHO and HEK stable cell line development
Bioprocess Development
High Throughput Protein Production
Tox Material
cGMP Cell Bank Manufacturing
OUR TECHNOLOGIES
Out-licensing of
superior cell hosts
In-house state-of-the-art tech
revolutionizing your development programs.
CHOExpress®
hosts cells
The fastest growing CHO host for biotech
We developed a uniquely high-performing production host system for large-scale manufacturing. CHOExpress® derivative clonal populations commonly show fast growth to densities over 20 million cells/mL, process yields over 5 g/L, and great robustness for high-scale manufacturing (1oo doublings).

The cell line is the result of nearly a decade of work, and is validated with FDA and EMEA cell line documentation, including TSE, BSE and mycoplasma-free statements. The cell line is immortalized.
CHOExpress® is highly compatible with other systems, and can be used royalty-free. ExcellGene will guide you in ensuring favorable phenotypes in CHOExpress®-derived clonal populations. Our knowledge on gene transfer and isolation of top-performing cell populations is at your disposal.
FlexiCHO® medium.
A high performance chemically defined medium formulation for growth and production and for biosimilar quality adjustements.
HEKExpress®
hosts cells
Pioneering single-cell suspension for transient gene expression and virus like particle production
HEKExpress® is a fast-growing, productive and robust HEK-293 derived cell line for large-scale manufacturing of recombinant proteins.
After nearly a decade of work with the adherent cell line, ExcellGene has generated an aggregate-free single-cell suspension culture with very high transfection efficiency. This also means scale-up in serum-free, animal component-free or chemically defined media is easy.
HEKExpress® demonstrates the highest TGE yield ever reported in the literature (> 1g/L). HEK-293 cells are commonly used for academic research, and are very suited for transient gene expression. The host system can be licensed along with our proprietary chemically defined medium.
Features
- A single-cell suspension host system for TGE and VLP production
- Highest transient expression yield ever reported in the literature (> 1g/L)
- Highly efficient in AAV9 production and lentivirus. Baculovirus-free
- Fully characterized, ready for cGMP master cell bank generation and cGMP production
- Single-cell suspension – no aggregates!
- Easy scale-up in various animal component free media types
- High cell density culture in ExcellGene’s CD media and feeds.
HEKzeroT® host cells
A clonally derived, T-antigen-free host cell system with superior AAV-VLP production capacity.
FlexiHEK® medium.
A high performance chemically defined medium formulation for growth and production for recombinant proteins.
BIOSIMILARS
cGMP-compliant clonal cell banks.
Exceptionally high productivity.
Flexible deal terms.
Proprietary CHOExpress® transposon-based technology with clinical and commercial (BLA) track record.
Benefit from our dynamic portfolio of biosimilars, de-risking your preclinical pipeline with seamless integration of upstream (USP) and downstream processes (DSP).
Ready-to-use (off-the-shelf)
- Highly productive, robust biosimilar clonal lines
- Research (RCB) and cGMP master cell banks (MCB)
- Manufacturing processes (USP, DSP) and respective analytics available
- Biosimilarity as defined by FDA/EMA guidelines
- Cost-effective solutions
Tailored on-demand
- Process development (USP, DSP)
- Biosimilarity tailor-made to market demands
- Scale up up to 200L
- Analytical packages
- Characterization of MCBs
- cGMP cell bank ready in less than 11 months
Top clone RCB titer up to 8 g/L,
98% of clones are stable
Cell culture protocols and
CMC-compliant documentation included for all cell banks
Critical quality attributes (CQAs) based on multiple reference lots including but not limited to:
Glycan profile,
Charge distribution,
Purity
Our biosimilar pipeline
Available for buy-out or licensing with regional or global rights.
Biosimilars generated from our cell lines consistently match critical quality attributes of the originator lots, ensuring identical performance and efficacy in the clinical trials with robust process scalability.
Glycoprofile Analysis
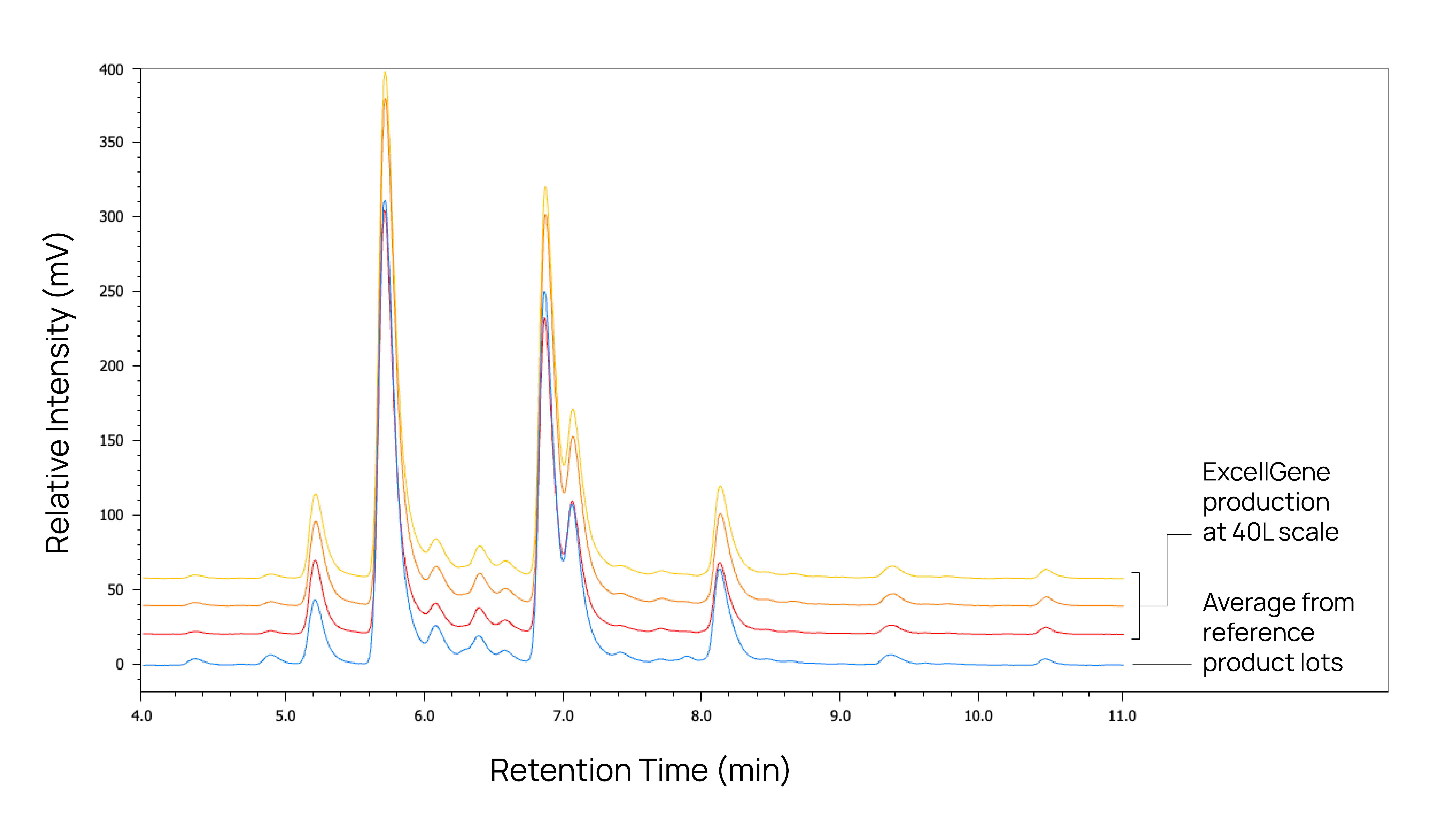
Explore our case study showcasing the development of a biosimilar until a cGMP master cell bank, including multiple successful confirmational runs at 40L.
Request a free quote for your project today.
From DNA to product: do it right, from the start.
